A lot more importantly, it is vital in verifying if cleaning processes are literally effective in stopping contamination.
I might use Individuals, Any time attainable, as single use. Alternatively, a rinse sampling is usually carried out for the hoses and for your brushes submerging them inside a container and having the media sample out from the container can be an alternative.
Before the cleaning validation limit is assessed and utilized, an analytical method with ample sensitivity, specificity, and recovery needs to be made and validated. The sampling in the cleaned floor with an appropriate swab product or rinse solvent is a vital future move to calculate the cleaning validation limit.
Description of device/devices/place:
The Performing social gathering would typically contain the next personnel associates, if possible All those with a superb insight into the company's operation.
g. sixty - a hundred in2, is wiped that has a sterile swab. The swab is then aseptically transferred to your sterile tube that contains a suitable diluent. The tube is then agitated to suspend any viable microorganisms and aliquots are put in a very semisolid medium to get quantitative outcomes.
Coupon may not be agent of equipment contamination or cleaning as it is actually independent from primarily floor.
• involving batches in strategies (when the very same components is being manufactured around a period of time, and on various times);
During the current get the job done, an industrial has taken some technique regarding to cleaning. The procedure was discovered to be validated as cleaning validation. All the equipments were selected from cross contamination standpoint based upon the matrix strategy. From this review, it might be concluded that cleaning validation is an important facet in assuring the substantial degree of assurance to your product or service quality.
The acceptance conditions, such as the rationale for setting the precise limits; Other items, processes, and equipment for read more which the prepared validation is legitimate in accordance to the “bracketing” thought; and
The volume of rinsing solvent applied is about 15-20ml but the selection of quantity is based on sort of sampling technique and analytical method made use of
Description of device/devices/spot:
The development, execution, and validation from the CVLM offers a responsible Software to simplify and automate the cleaning validation calculations to guidance a compliant cleaning validation plan.
A proper cleaning method validation will enhance the process of the corporation’s machines cleaning and may absolutely free the corporate from experiencing authorized actions for not accomplishing it. Hence just about every corporation the place a pharmaceuticals or in any respect industries it operates in should here always notice this process.
 Emilio Estevez Then & Now!
Emilio Estevez Then & Now!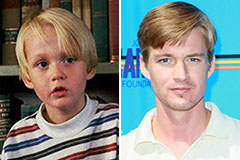 Mason Gamble Then & Now!
Mason Gamble Then & Now! Joshua Jackson Then & Now!
Joshua Jackson Then & Now! James Van Der Beek Then & Now!
James Van Der Beek Then & Now! Batista Then & Now!
Batista Then & Now!